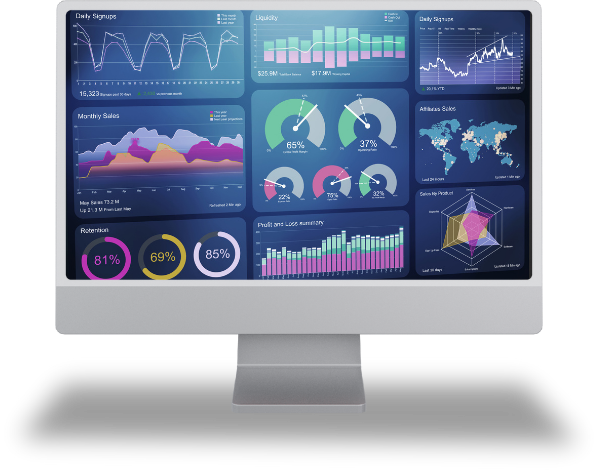
The PM Dashboard provides a real-time, centralised view of clinical trial progress across studies, countries, and sites. Designed for late-phase clinical trials, it brings together critical project data into a single, intuitive interface—enabling sponsors, CROs, and project teams to monitor timelines, milestones, risks, and dependencies with confidence. By delivering timely insights and complete transparency, the PM Dashboard supports proactive control, regulatory compliance, and on-time study delivery.
Gain complete visibility across complex, multi-site clinical trials from a single platform. Maintain control with real-time tracking, audit-ready data, and structured governance.
Access real-time project insights to identify risks, dependencies, and delays early. Enable proactive decision-making that keeps trials moving without last-minute pressure.
Reduce manual follow-ups, handoffs, and coordination across study teams. Streamline workflows to minimise delays and improve overall trial productivity.
Keep sponsors, CROs, and study teams aligned with shared data and dashboards. Ensure consistent communication and accountability across all trial stakeholders
Execute trials on time with compliance embedded into every process. Support regulatory requirements through controlled workflows and traceable actions.
Stay on track with clear milestones, progress visibility, and risk indicators. Increase confidence in meeting study timelines and critical trial objectives.






faster identification and resolution of project risks and delays
reduction in manual status reporting effort across cross-functional teams
improvement in on-time milestone delivery and schedule adherence
traceability of project activities to support inspections and regulatory audits
6th Floor, Arista, Anandnagar road, Ahmedabad 380015, India